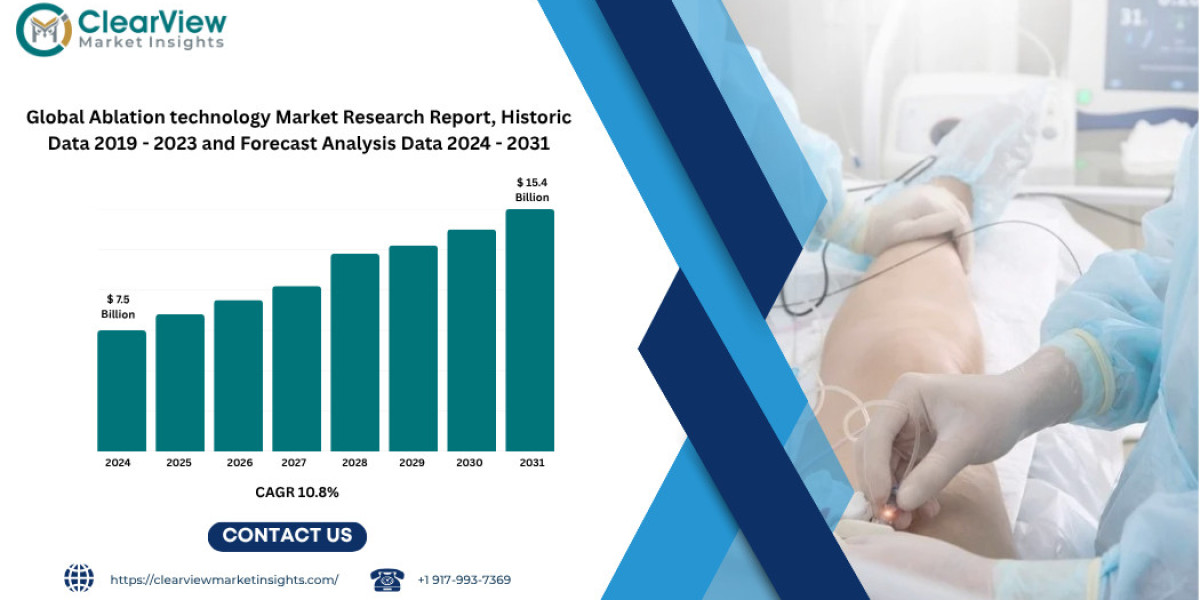
Chicago, 2nd june 2025 — Clearview Market Insights (CVMI) forecasts that the global ablation technology market will grow from USD 7.5 billion in 2024 to USD 15.4 billion by 2031, reflecting a robust compound annual growth rate (CAGR) of 10.8 percent. Demand is driven by surging atrial fibrillation (AF) caseloads, rapid adoption of minimally invasive oncology treatments, and breakthrough energy modalities such as pulsed field ablation (PFA) and high intensity focused ultrasound (HIFU).
“Energy‑based ablation is reshaping how clinicians treat arrhythmias, tumors, chronic pain, and more,” said Dr Alexandra Hart, Interventional Devices Lead Analyst at CVMI. “With AI‑guided mapping, robotics, and non‑thermal tissue‑selective pulsed fields, safety profiles are improving while procedure times drop—opening the door to broader outpatient adoption.”
Request Sample @ https://clearviewmarketinsights.com/report-details/global-ablation-technology-market/
Key Figures
Metric | 2024 | 2031 | CAGR (2024‑31) |
Market Size (USD B) | 7.5 | 15.4 | 10.8 % |
Cardiac Electrophysiology Share | 46 % | 48 % | — |
Oncology Ablation Share | 32 % | 31 % | — |
Asia‑Pacific CAGR | — | 13.1 % | — |
Growth Catalysts
- Aging & AF Epidemic — Estimated 12 million AF patients in the U.S. by 2030; catheter ablation adoption rises.
- Next‑Gen Energy — PFA catheters show single‑shot pulmonary‑vein isolation in <15 minutes with low esophageal risk.
- Oncology Shift — Liver, kidney, lung, and bone tumors increasingly treated percutaneously with microwave and cryo technologies.
- Robotics & AI — Contact‑force sensors and AI mapping reduce fluoroscopy time and improve lesion durability.
- Outpatient Migration — Ambulatory surgery centers capture rising share of pain and thyroid ablation procedures.
Leading Players
Company | 2024 Share | Recent Highlight |
Medtronic | 22 % | CE Mark for PulseSelect™ PFA; global launch in progress |
Boston Scientific | 18 % | Completed FARAPULSE IDE; U.S. approval expected 2025 |
Johnson & Johnson – Biosense Webster | 16 % | Opti‑PFA catheter pilot in EU; CARTO™ AI mapping upgrade |
Abbott | 11 % | FDA clearance for FlexAbility™ SE with contact‑force feedback |
AngioDynamics | 6 % | NanoKnife™ electroporation prostate IDE underway |
Regional Snapshot
- North America — Largest revenue share (43 %); CMS adds outpatient AF ablation codes.
- Europe — Early PFA adoption in Germany, UK; robust HIFU therapy for uterine fibroids.
- Asia‑Pacific — Fastest growth; China expands microwave liver‑tumor programs, Japan subsidies cardiac PFA.
- Latin America — RF and cryo dominate; Brazil funds public‑sector pain‑ablation centers.
- Middle East & Africa — Gulf cardiac institutes pilot robotic RF labs; oncology HIFU adoption begins.
2024‑25 Milestones
Quarter | Event | Effect |
Q4 2023 | Medtronic PulseSelect™ PFA CE Mark | First non‑thermal AF system approved in EU |
Q2 2024 | Boston Scientific completes FARAPULSE IDE | Sets stage for 2025 FDA submission |
Q3 2024 | AngioDynamics starts NanoKnife™ prostate IDE | Opens new urologic application vertical |
Q4 2024 | Chinese CFDA fast‑tracks domestic HIFU console | Boosts local oncology capacity |
Q1 2025 | Abbott FlexAbility™ SE U.S. clearance | Expands contact‑force RF portfolio |
Technology Trends
- Pulsed‑Field Ablation — Non‑thermal electroporation selectively targets myocardium, reducing collateral damage risk.
- Robotic Navigation — Robotic arms stabilize catheters, cutting radiation exposure by up to 40 %.
- AI Lesion Assessment — Real‑time algorithms quantify lesion depth and predict reconnection gaps.
- Hybrid Ablation Suites — Combined RF‑microwave systems treat multifocal liver tumors.
- Eco‑Smart Disposables — Recyclable catheter housings and gas‑free cryo alternatives reduce environmental impact.
Looking Forward
- 2025 — U.S. approval of first PFA system triggers wave of outpatient AF centers.
- 2026 — HIFU receives FDA clearance for pancreatic tumor palliation.
- 2027 — AI‑guided lesion durability metrics standard in EU electrophysiology labs.
- 2028 — Single‑use robotic arms enter oncology ablation, slashing capital barriers.
- 2029 — Global electroporation oncology revenues surpass USD 1 billion.
- 2030 — Pulsed‑field systems account for 40 % of new AF ablation installs.
- 2031 — Over 70 % of liver and kidney ablations performed in ambulatory settings.
For more insights, visit https://clearviewmarketinsights.com/
About Clearview Market Insights:
Clearview Market Insights is a leading market research and consulting firm providing in-depth industry analysis and strategic recommendations for businesses worldwide.
Media contact:
Bhavani K
Marketing and Sales Head
ClearView Market Insights
Mail: sales@clearviewmarketinsights.com
Phone: +1 917-993-7369